Implementation of a Canadian Prescription Drug Importation Program

In accordance with Senate Bill 19-005, passed in 2019, the Department of Health Care Policy & Financing (HCPF) is developing a Canadian prescription drug importation program. The Federal Food Drug and Cosmetic Act (FDCA) Section 804 permits importation of prescription drugs from Canada by a pharmacist or wholesaler, provided the drugs meet certain minimum standards, will pose no additional risk to the public's health and safety, and results in a significant reduction in costs to consumers. HHS can approve a program if these conditions are met.
Program Overview
Senate Bill 19-005, which directs the Department of Health Care Policy & Financing (HCPF) to implement a Canadian Prescription Drug Importation Program, was signed into law by Governor Jared Polis. In December of 2019, the federal Department of Health and Human Services (HHS) released a Notice of Proposed Rulemaking, or the draft rule. In response, Colorado responded with a program proposal and extensive comments in March of 2020, in hopes of seeing Colorado’s requested changes updated in a final version of the rule. The final rule went into effect on November 30, 2020, putting in place the federal regulatory framework to successfully develop and operate an Importation Program. After taking the provisions of the Final Rule into account, HCPF released an Invitation to Negotiate (ITN) in January 2021, seeking vendors for the program and announced all partners in August 2022. In December 2022, HCPF submitted its original Section 804 Importation Program (SIP) application to the federal Food and Drug Administration (FDA) for review. In response to FDA requests for additional changes, HCPF submitted an amendment on December 5, 2025. HCPF estimates that the Colorado Importation Program will be approved in 2026.
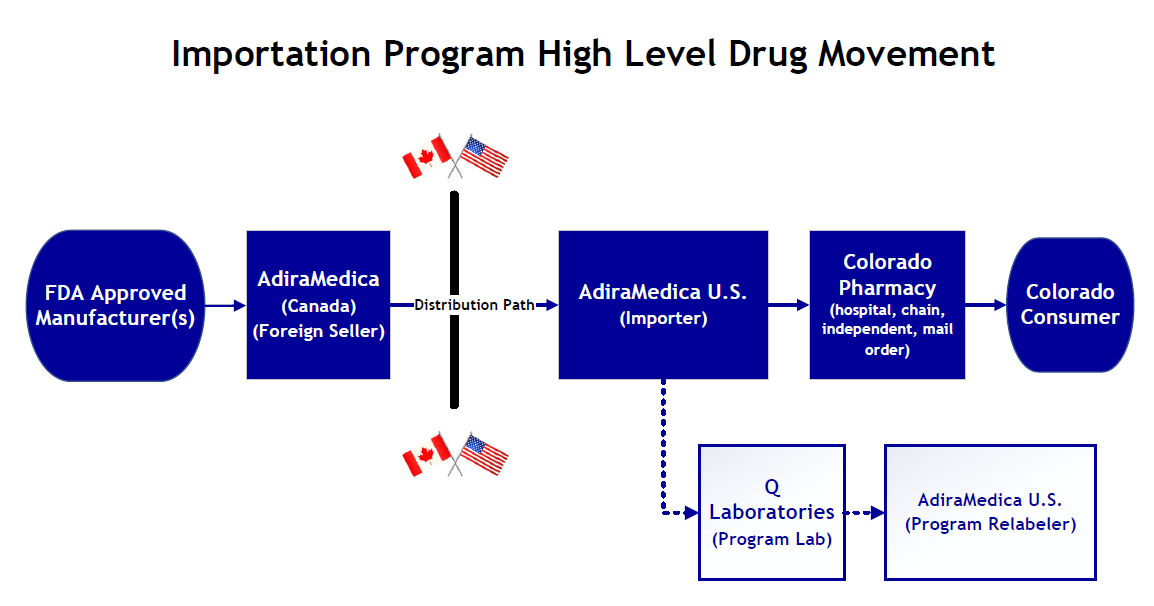
FDA approved manufacturers will sell the eligible prescription drugs to Colorado’s Foreign Seller, AdiraMedica, which is located in Canada. AdiraMedica will export the eligible prescription drug to an AdiraMedica subsidiary located in Pennsylvania. The imported medications will then be sent to Q Laboratories for testing. Once the tests are approved by FDA, the imported drugs will be relabeled by Adira and then can be distributed to participating Colorado pharmacies where they can be dispensed to Colorado consumers.
- Colorado's Canadian Drug Importation Program Frequently Asked Questions
- Importation Vendor Fact Sheet
- Manufacturer Fact Sheet
Annual Report to the Colorado General Assembly
Colorado Section 804 Importation Program (SIP) Application
2025 Application Documents
- Amended SIP Application
- Amended SIP Appendices
Resources
- Colorado's Canadian Drug Importation Program Frequently Asked Questions
- Manufacturer Fact Sheet
- Importation Vendor Fact Sheet
- Vendor Notice - November 2025
- Colorado Response for MAHA AHRQ RFI - July 2025
- FDA Takes Steps to Enhance State Importation Programs to Help Lower Prescription Drug Prices - May 2025
- Delivering Most-Favored-Nation Prescription Drug Pricing To American Patients - May 2025
- Colorado Response for Section 232 Investigations of Pharmaceutical Tariffs - May 2025
- Colorado OMB Deregulation Request for Information for the Importation Final Rule - May 2025
- Lowering Drug Prices By Once Again Putting Americans First - April 2025
- Letter from FDA Commissioner Califf to Governor Jared Polis - January 2025
- Food and Drug Administration Request For Information - December 2024
- Colorado Citizen Petition Response to PhRMA (Docket No.2023–P-1773-004) - July 2024
- FDA Request For Information to SIP Application - March 2023
- Executive order on Promoting Competition in the American Economy - July 2021
- Drug Importation in Colorado International Pricing Report - January 2021
- Importation Program Solicitation Press Release - January 2021
- Final Rule: Importation of Prescription Drugs - November 2020
- FDA Takes Action to Help Lower U.S. Prescription Drug Prices - September 2020
- Executive Order Increasing Drug Importation to Lower Prices for American Patients - July 2020
- Safe Importation Action Plan - July 2019
- SB 19-005 Canadian Prescription Drug Importation Act - May 2019
- Federal Statute Regarding Canadian Drug Importation (21 U.S.C. 384) - 2003
Stakeholder Opportunities
- The Department held a virtual stakeholder meeting on March 12, 2024.
Stay Informed
We encourage you to sign up for updates on our drug importation work. Please fill out the signup form to be added to our list.
We can also be contacted via email at HCPF_005DrugImportation@state.co.us.